Regulatory Compliance Requirements in Healthcare App Development
Core Insights
Developing a healthcare app in 2025 means navigating an increasingly complex regulatory landscape. With sensitive health data at stake, organizations must comply with strict frameworks that govern how information is collected, stored, shared, and protected. Whether you're building a telemedicine platform, a fitness tracker, or a clinical decision support tool, your app is likely subject to regulations such as HIPAA (for U.S. users), GDPR (for users in the European Union), FDA oversight (if your app is considered a medical device), and global quality standards like ISO 27001 and ISO 13485.
These laws and standards ensure your product is trustworthy, ethically sound, and ready for real-world use. In this article, we break down the most important regulatory compliance requirements for healthcare app development and provide actionable strategies to help your solution remain secure, compliant, and future-proof.
Why Regulatory Compliance Is Critical in Healthcare App Development
The healthcare industry processes some of the most sensitive personal information – medical history, diagnoses, prescriptions, biometric data, and more. As digital solutions continue to replace paper-based systems, the responsibility to protect this data falls heavily on healthcare app developers.
Regulatory compliance ensures that apps handling health information follow strict privacy, security, and ethical standards. But beyond legal obligations, compliance is a cornerstone of trust and clinical safety.
Key Risks of Non-Compliance:
- Legal and Financial Penalties: Regulatory bodies like HHS OCR can impose fines ranging from thousands to millions per violation.
- Reputational Damage: A single privacy incident can destroy patient trust and user loyalty.
- Market Access Issues: App stores such as Google Play and the Apple App Store now enforce strict privacy requirements, especially for health-related apps.
- Clinical Impact: Faulty or non-compliant apps can result in misdiagnoses, poor outcomes, or even patient harm.
Why It Matters
Regulatory compliance is something more than just a legal checkbox. Nowadays, it’s a strategic investment. It helps you:
- Build user trust
- Shorten sales and approval cycles
- Avoid costly rework
- Ensure your product is clinically safe and ready for scale
Healthcare apps that treat compliance as a foundation are more likely to succeed in both regulated and consumer-facing markets.
Key Regulatory Frameworks You Must Understand
Different regulations apply depending on the location of your users, the type of data you collect, and whether your app has clinical functionality. Here's a breakdown of the four most important frameworks that shape healthcare app development today.
HIPAA – Health Insurance Portability and Accountability Act (USA)
If your app collects or processes Protected Health Information (PHI) in the U.S. and works with healthcare providers or insurers, HIPAA compliance is mandatory.
HIPAA Compliance Checklist:
- Implement encryption for PHI in transit and at rest
- Enforce Role-Based Access Control (RBAC)
- Set up audit logs for data access and changes
- Ensure proper authentication (e.g., multi-factor)
- Sign Business Associate Agreements (BAAs) with all third-party vendors handling PHI
HIPAA is enforced by the U.S. Department of Health and Human Services (HHS) and violations can lead to fines up to $1.5 million per year per violation category.
GDPR – General Data Protection Regulation (EU)
If your app is available to users in the European Union, or even collects data from EU citizens, you must comply with the GDPR.
Core GDPR Principles for Healthcare Apps:
- Obtain explicit and informed user consent
- Offer data access, correction, and deletion (right to be forgotten)
- Limit data use to its stated purpose
- Minimize data collection to what is strictly necessary
- Provide data portability options
GDPR fines can reach €20 million or 4% of global annual revenue, whichever is higher.
FDA Oversight – Software as a Medical Device (SaMD)
Apps that provide diagnosis, treatment planning, or clinical support may be classified as Software as a Medical Device (SaMD) and fall under FDA regulation.
Apps That May Require FDA Clearance:
- Diagnostic tools (e.g., skin lesion analyzers)
- Apps that calculate drug dosages
- Smartphone-enabled ECG monitors
- AI-based clinical decision support tools
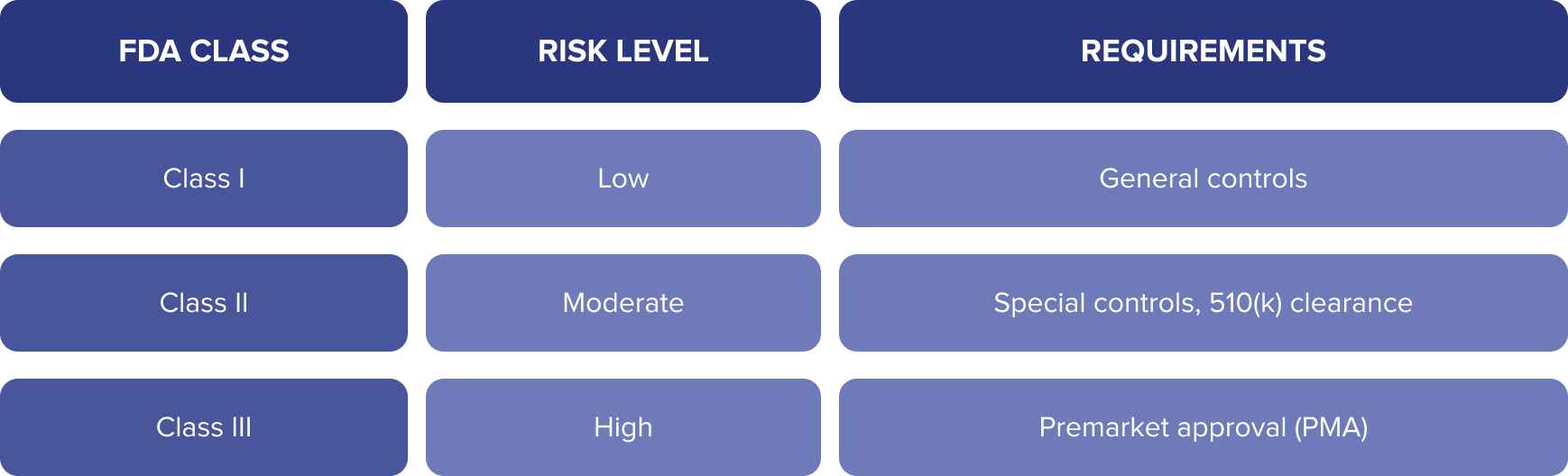
Use the FDA Digital Health Policy Navigator to determine if your app qualifies as a medical device.
ISO Standards – Global Quality & Security Guidelines
While ISO standards aren’t laws, they’re globally recognized frameworks that can help your healthcare app demonstrate security, quality, and readiness for regulated markets.
Key ISO Standards:
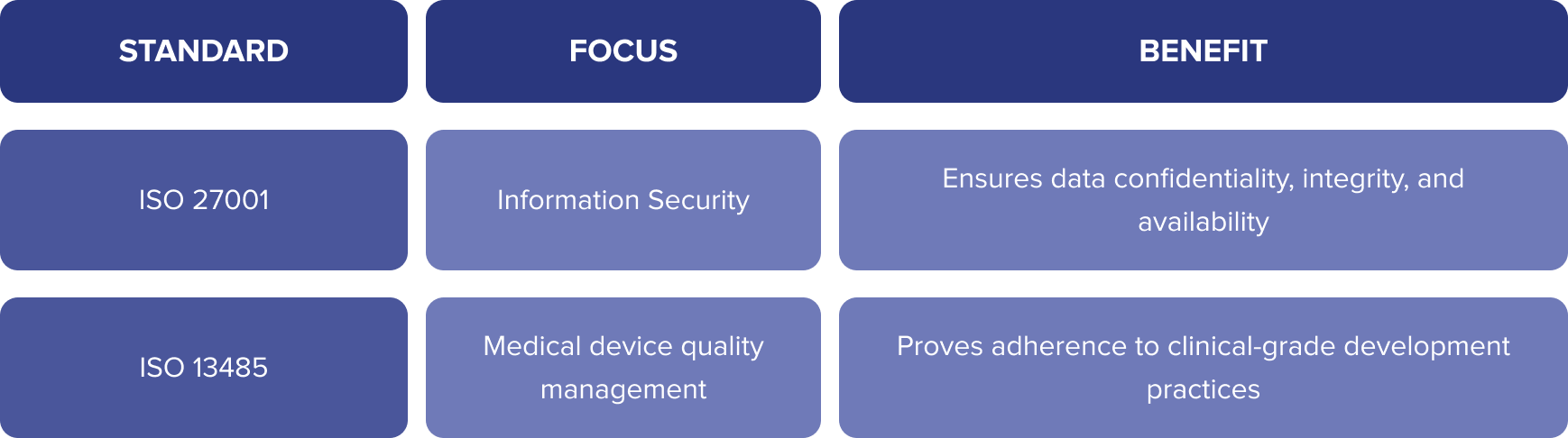
Benefits of ISO implementation:
- Boosts market credibility
- Aligns with GDPR, HIPAA, and FDA expectations
- Prepares your app for audits and B2B procurement
Don’t Overlook Additional Regulations
While HIPAA, GDPR, FDA, and ISO cover the core of healthcare compliance, several regional and domain-specific regulations can also apply – especially if your app is used across multiple states or countries, or if it handles payments, emergency services, or government programs.
State-Level Privacy Laws (U.S.)
Not all patient data is covered by HIPAA. Apps outside traditional provider settings may be subject to state privacy laws, which are increasingly stricter.
Examples:
- CPRA (California): Expands on CCPA. Requires “Do Not Sell” options, granular consent, and detailed user rights.
- Colorado Privacy Act (CPA), Virginia CDPA, Connecticut, Washington: Introduce consent, access, and deletion rules similar to GDPR.
Tip: Design your app with configurable consent and data portability features to comply across regions.
CMS Conditions of Participation (USA)
If your app integrates with Medicare or Medicaid providers, it must align with CMS guidelines. These include:
- Secure EHR integrations
- Emergency treatment compliance (see EMTALA)
- Interoperability standards (FHIR)
EMTALA: Emergency Medical Treatment and Active Labor Act
If your app facilitates urgent care or ER triage, you may need to comply with EMTALA, which prohibits “patient dumping” and mandates stabilization before transfer or discharge.
PCI DSS: Payment Security
Apps that process credit/debit card transactions must comply with PCI DSS, a security standard for financial data. This includes:
- Secure payment gateways
- Tokenization
- Strict access controls for payment data
Global Privacy & Security Laws
- PIPEDA (Canada): Consent-driven regulation for personal data
- UK DPA 2018: Post-Brexit GDPR equivalent
- Australia’s Privacy Act: Includes Notifiable Data Breach (NDB) scheme
Actionable Steps to Ensure Regulatory Compliance
Achieving compliance in healthcare app development is an ongoing, structured process. Whether you’re building a telehealth app, a chronic condition tracker, or a clinical decision support tool, the following steps will help ensure your product aligns with the relevant laws and standards from day one.
1. Conduct a Regulatory Risk Assessment
Before writing any code, identify:
- Which laws apply (HIPAA, GDPR, CPRA, FDA, ISO, etc.)
- What types of data you’ll collect (PHI, biometrics, payment info)
- Where your users are located
This foundational step will guide every design and development decision.
2. Build With Privacy by Design
This principle means compliance is baked into the architecture.
Best Practices:
- Enable default privacy settings (opt-in, not opt-out)
- Use data minimization: collect only what’s essential
- Separate user roles and control access based on need
- Allow users to withdraw consent or delete their data at any time
Learn more about Privacy by Design.
3. Map Data Flows and Secure PHI
Create a visual map or flowchart of:
- Where PHI enters the system
- How it’s stored, transmitted, and deleted
- Which third parties have access
Apply end-to-end encryption, secure APIs, and role-based access controls (RBAC) at every stage.
4. Collaborate With Legal and Compliance Experts
Partner with:
- Healthcare lawyers
- Privacy consultants
- Security auditors
They help interpret complex laws, prevent oversights, and support certification or approval processes.
5. Document Everything
Keep detailed records of:
- Consent forms and user permissions
- Access logs and audit trails
- Risk assessments and test results
- Staff compliance training
This is especially important for passing audits or demonstrating due diligence after a breach.
6. Maintain and Monitor
- Regularly test for vulnerabilities (e.g., penetration testing)
- Patch systems promptly
- Stay updated on evolving regulations
Tip: Use automated compliance monitoring tools (e.g., for HIPAA or GDPR) to flag gaps early.
Building Trust Through Security & Transparency
In healthcare, compliance is a powerful trust signal. Users expect that their health data will be treated with the same care as their in-person medical records. When you demonstrate strong security and transparency practices, you earn user confidence, encourage adoption, and improve retention.
Security Signals That Build Confidence
- End-to-end data encryption
- Multi-factor authentication (MFA) for login and data access
- Clear permissions prompts when collecting sensitive data
- Access logs and user-visible activity history
- Secure cloud or on-premise storage partners that meet ISO/HIPAA standards
Tip: Display security badges and certifications (e.g., HIPAA Compliant Hosting, ISO 27001 Certified) prominently in your app or landing page.
Transparency Features Users Expect
- A plain-language privacy policy that explains:
- What data is collected
- Why it’s collected
- How it’s stored, shared, and deleted
- What data is collected
- In-app access to download or delete personal data
- A visible consent management dashboard
- Real-time alerts about data sharing or breaches
According to Pew Research, over 79% of Americans are concerned about how companies use their health data. Providing control, visibility, and assurance can turn privacy into a competitive advantage.
Future-Proofing Your App for a Changing Regulatory Landscape
New technologies like AI, wearable devices, and cross-border data flows are driving continuous changes in how laws are interpreted and enforced. To remain competitive, your app must be built on an adaptable compliance strategy that evolves as the landscape does.
Key Strategies for Long-Term Compliance
1. Modular, Scalable Architecture
Use microservices, API-first development, and isolated compliance components (e.g., consent management, audit logging) that can be updated independently of core features.
2. AI & SaMD Governance Readiness
If your app uses machine learning or qualifies as a Software as a Medical Device (SaMD):
- Maintain explainability (transparency of AI decisions)
- Prepare for FDA/EMA algorithm review frameworks
- Log and store clinical logic or model inputs/outputs
3. Continuous Monitoring
Set up systems to:
- Track regulatory updates
- Flag emerging risks (e.g., through automated compliance tools)
- Trigger alerts when key standards (HIPAA, GDPR, ISO) evolve
4. Proactive Updates & Patch Management
- Automate deployment pipelines for faster security patches
- Schedule regular compliance audits, especially when launching new features
- Monitor and update third-party SDKs, especially those handling sensitive data or analytics
Building compliance into your foundation is the only way to scale your product across markets while keeping regulators, partners, and users on your side.
Turning Regulations into Opportunity in Healthcare App Development
Navigating healthcare compliance might seem daunting, but at the same time, it’s a strategic advantage. By aligning with HIPAA, GDPR, FDA, and ISO standards, you’re not just reducing legal risks – you’re building trust, accelerating time-to-market, and preparing your app for global scalability.
In 2025 and beyond, the most successful healthcare apps will be those that embrace compliance as a foundation for innovation. Whether you're building an AI-powered diagnosis tool, a telemedicine platform, or a patient engagement app, security, transparency, and ethical design are non-negotiable.